Pioneering endosurgery
Since 2017, Lumendi has worked with gastrointestinal and colorectal practitioners to make complex polypectomies and other GI interventions faster, safer, and less invasive with our innovative DiLumen® Endoluminal Interventional Platform (EIP).
We’re committed to expanding possibilities in endosurgery—helping physicians perform at a higher level and minimize risk and recovery time for patients.
Our products put more possibilities for endosurgery within reach.
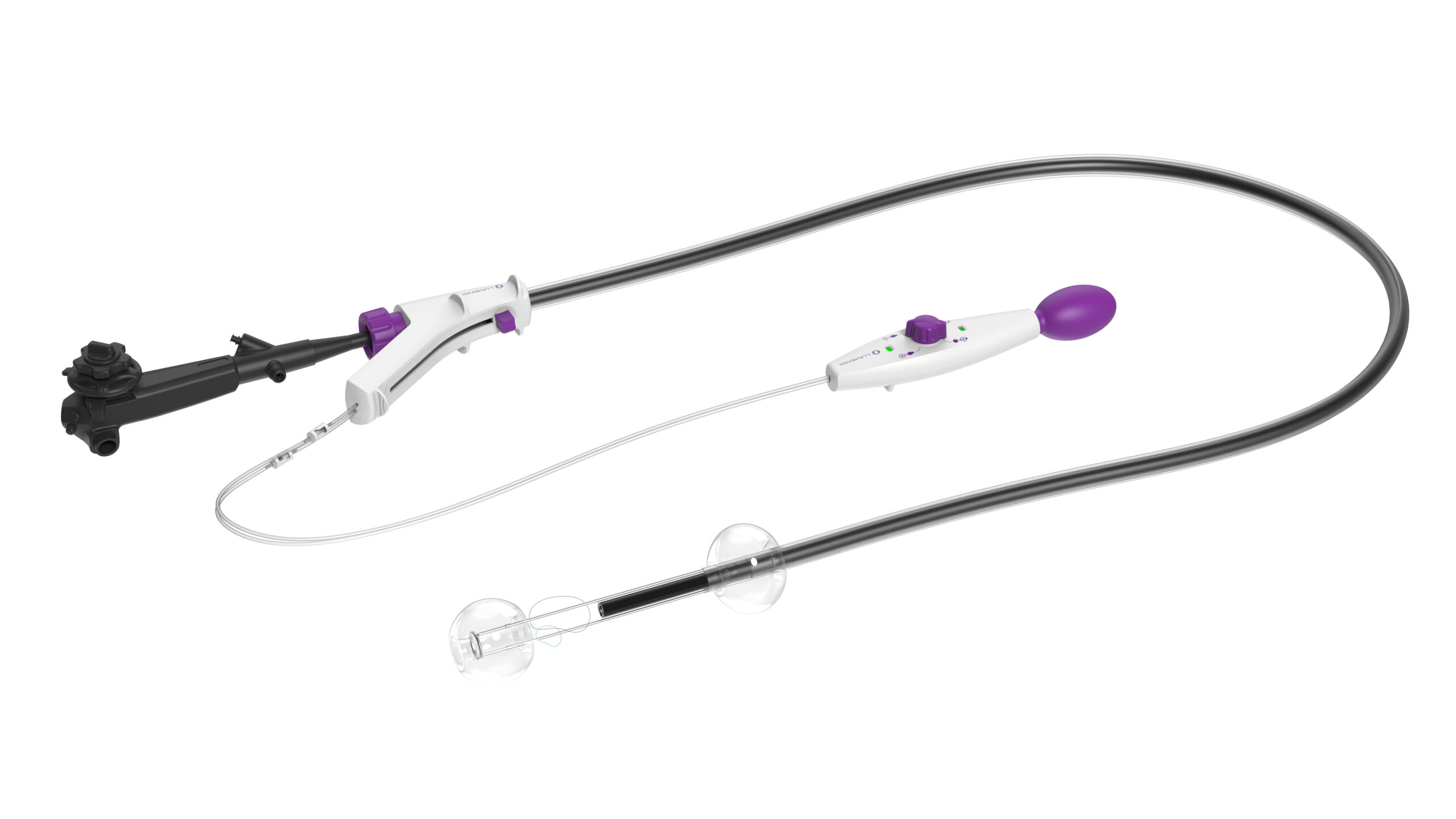
DiLumen EZ Glide
Enhances EMR, ESD, complex polypectomies, and challenging colonoscopies with superior navigation and improved stability and control.
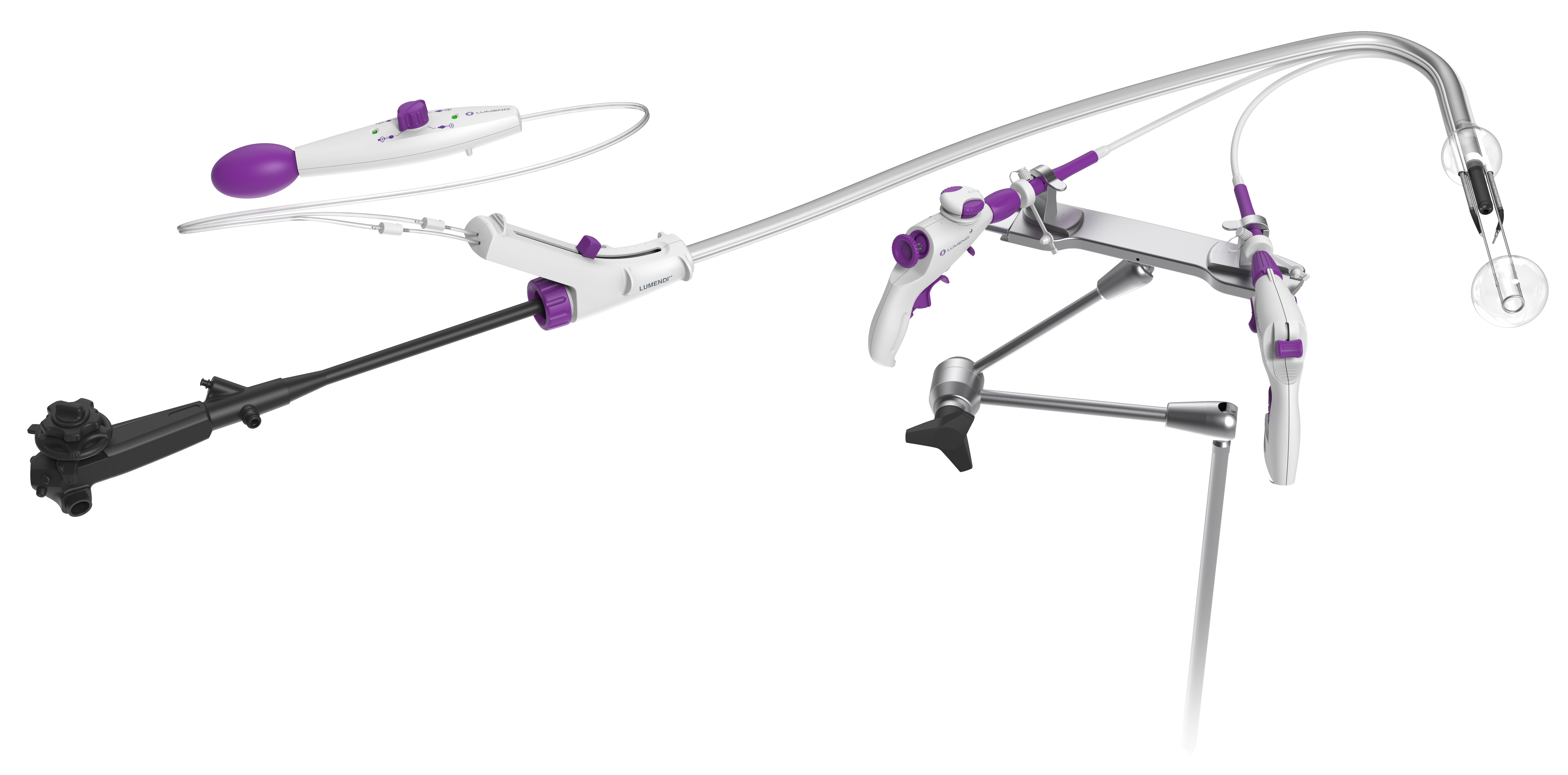
Learn MoreDiLumen C²
Enabling unprecedented tissue manipulation via robotic-like end effectors to endoluminal surgery in a lower-cost, disposable format.
Learn moreOur latest news & events:
EZ¹ successfully completes Customer Evaluation phase.
Lumendi Achieves Key Milestone with Completion of DiLumen EZ¹ Customer Evaluations, Setting the Stage for the Next Phase of DiLumen Product Innovation WESTPORT, CT – July 27, 2023 Lumendi, a… Continue reading EZ¹ successfully completes Customer Evaluation phase.
Read MoreA successful Digestive Disease Week (DDW) conference in San Diego
Since the company’s inception, we have accumulated over 100 DiLumen users worldwide. Half of these physicians have begun using our devices in the last 12 to 18 months. This year’s DDW was our first opportunity in three years to present Lumendi and our significantly enhanced DiLumen platform to users and industry.
Read MoreLumendi Reports Successful Completion Of Dilumen™ Eip First-in-human Trials
LUMENDI REPORTS SUCCESSFUL COMPLETION OF DILUMEN™ EIP FIRST-IN-HUMAN TRIALS Primary Study Endpoint Reached with no Serious Adverse Events WESTPORT, CONNECTICUT, November 16, 2017 – Connecticut-based medical device innovator Lumendi,… Continue reading Lumendi Reports Successful Completion Of Dilumen™ Eip First-in-human Trials
Read More30% of surgical interventions are unnesessary
Dr. Peter Johann, CEO & Founder of Lumendi and Prof. Bauerfeind discussed how treatment methods such as EMR and ESD can be made more accessible to patients. Prof. Bauerfeind: "Sometimes these treatments… Continue reading 30% of surgical interventions are unnesessary
Read MoreExpand your knowledge of what’s possible in endosurgery with Lumendi.
Leave us your information to stay in touch.